First Lecture: (Video) - BLACKBOARD
Second Lecture: (Video not available) - BLACKBOARD
Third Lecture: (Video not available) - BLACKBOARD



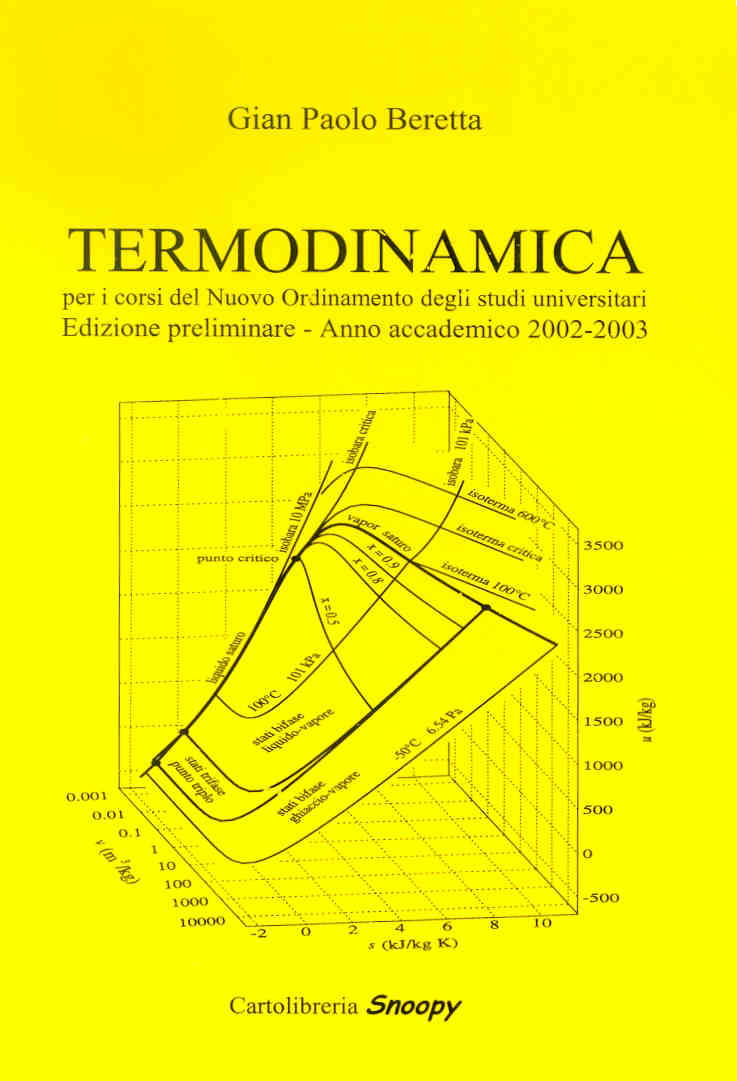 **
**
MIT-IAP 2008
Course Outline (3 x 2hr lectures)
This short course is open to students and teachers who wish to take a quick view (review) at how the general principles of thermodynamics can be very effectively presented and taught in a rigorous way, by following the treatment developed in the textbook
Gyftopoulos & Beretta, Thermodynamics. Foundations and Applications (originally published by Macmillan in 1991, now reprinted by Dover in 2005, with problem solutions). The treatment resolves conceptual loopholes and logical deficiencies which are present in traditional treatments of thermodynamics, and it is rigorous because it builds up a set of unambiguous definitions of all the basic concepts needed in the exposition (system, property, state; nonequilibrium, equilibrium, and stable equilibrium state; process, weight process, reversible process; thermal reservoir).
The book has had a scientific role in the development of thermodynamics, particularly because it has provided the first noncircular definition of entropy, valid also for nonequilibrium states. It adopts the statement of second law introduced by Hatsopoulos and Keenan in 1965, i.e., the assertion of existence of a unique stable equilibrium state for each set of values of the energy, the amounts of constituents, and the volume (and/or other parameters), from which, of course, all the results of classical thermodynamics unfold as rigorous results. But the simple and general extension to nonequilibrium -- absent from traditional expositions and provided here avoiding intentionally any statistical reasoning – makes the approach timely and relevant for a variety of applications at the frontiers of the many fields of science and engineering where nonequilibrium is the keyword. Moreover, by introducing the ‘simple system’ model the book clearly separates the concepts, results, and relations that hold for all well-defined systems, including microscopic systems, from those which hold only for macroscopic and mesoscopic systems, another restriction of traditional expositions.
The short course will illustrate how to present the essential concepts in a small number of lectures suitable for an introduction at the undergraduate level or an introductory review at the graduate level. Proofs and details can be skipped because the students may find them in the book, if they wish, but the important aspect remains that the approach is rigorous and logically unambiguous. Because neither the students nor the teachers need to be puzzled by the typical logical difficulties and ambiguities of the traditional expositions (entropy defined in terms of heat and temperature, heat and temperature defined in terms of entropy), they can all proceed quickly and safely to the applications of interest.
Detailed outline
definitions of system, property, state, process, weight process
first law of thermodynamics and its consequences: definition of energy, additivity, echangeability, and conservation of energy, energy balance equation
definitions of reversible process, equilibrium state, unstable, metastable, and stable equiibrium state, thermal reservoir
Hatsopoulos-Keenan statement of the second law of thermodynamics, its main conceptual consequences
definition of temperature of a thermal reservoir,
definition of entropy valid for all states (including non-equilibrium), additivity, exchangeability, 'principle' of non-decrease of entropy in weight processes, entropy balance equation
the energy versus entropy diagram, a very effective tool for providing a graphical summary of the principles, for consistent reasoning and inference
stable equilibrium state theorem, fundamental stable-equilibrium-state relation S=S(E,n,V), maximum entropy 'principle' and minimum energy 'principle', Gibbs relation
definitions of temperature, pressure, chemical potentials and their significance and importance related to the necessary conditions for mutual stable equilibrium
adiabatic availability as an important property of nonequilibrium states
available energy with respect to a reservoir
definition of heat interactions (Yes! Heat is defined rigorously, and it is not used to define entropy!), work interactions, nonwork interactions different from heat, Carnot coefficient
definition of the simple system model (this is where the idea of macroscopic versus microscopic system comes in. Yes! Results up to here apply to all systems,including a single particle)
Euler relations and its consequences, Gibbs-Duhem relation, Gibbs free energy in terms of chemical potentials
bulk flow interactions, energy and entropy balance for a system with various kinds of interactions, exergy
Contact
instructor for further details. www.gianpaoloberetta.info
 Instructor: Gian Paolo
Beretta
Instructor: Gian Paolo
Beretta
** The text in italian is published by: Casa Editrice Cartolibreria SNOOPY s.n.c.,
via Bligny n.27 - 25133 Brescia - Tel. e Fax 0302006331, E-mail cartolibreriasnoopy@numerica.it